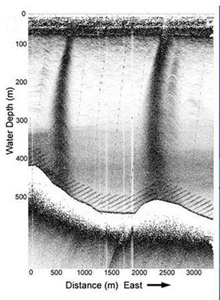
A sub-bottom profile image of a bubble plume rising from the sea floor. Click image for larger view and image credit.
A mass spectrometer with a uniquely designed membrane inlet system, allowing in situ dissolved gas measurements in deep-sea environments. Click image for larger view and image credit.
Why Study Dissolved Gases in the Deep Sea?
Peter Girguis
Harvard University
Scott Wankel
Harvard University
Each and every day, there is a continuous and dynamic exchange of gases and water between the atmosphere and the oceans. Ocean-atmosphere exchange affects the lives of animals, plants, and microbes that live both on land and in the oceans. For example, increases in seawater carbon dioxide concentration (due to natural or human causes) can impact ocean communities from the sea surface to the deep ocean. Identifying and quantifying dissolved gas concentrations in seawater can help us understand biological processes such respiration, photosynthesis, and the nitrogen cycle. By studying ocean-atmosphere exchange, we can understand the geochemical linkages which couple the terrestrial, oceanic, and atmospheric processes that govern the health of our biosphere.
In the deep-sea, dissolved gas concentrations can also be used for better understanding geological and biological processes. For example, dissolved gases help us model how superheated hydrothermal vent water mixes with the cold surrounding seawater. Studying dissolved gases, such as methane, allow us to investigate how microbes both produce and consume methane. Methane gas can also be used to help governments and companies find new sources of fossil fuels, including oil and natural gas, in the shallow and deep oceans.
There are major obstacles that must be overcome when studying dissolved gases in the deep-sea. Many laboratory instruments have trouble measuring gases dissolved in seawater. For example, gas chromatographs are adept at separating and quantifying a number of gases simultaneously but (as the name suggests) cannot measure gases in liquids. Some instruments, such as dissolved oxygen meters, are very good at measuring oxygen in seawater, but they are usually capable of only measuring one compound.
Sometimes researchers collect water samples and measure dissolved gases on the ship or back in the lab, but the longer you wait to measure them, the more the gas concentrations could change. Biological consumption (such as oxygen being used for respiration) or abiotic factors (such as offgassing due to changes in temperature or pressure) are a few reasons this may occur In addition, the laboratory analyses are so time consuming that the number of samples that can be realistically processed and analyzed is small. In deep-sea environments, these challenges can be even greater due to the nature of remote sampling under extreme pressures and temperatures.
Chemical mapping during this cruise will help us better understand the role dissolved gases play in the distribution of chemosynthetic animals, such as these mussels and tubeworms. Click image for larger view and image credit.
On this research cruise to deep hydrocarbon seeps in the Gulf of Mexico, we will be using an instrument called a mass spectrometer that is designed to quickly measure a number of dissolved gases at very specific points at the seeps, like right next to a mussel siphon or the end of a tubeworm tube. Previous data from the upper slope seeps (and other chemosynthetic communities as well) suggest the distribution of hydrocarbons, such as methane, ethane, butane and propane as well as hydrogen sulfide, play a primary role in determining which animal communities inhabit these environments. We will use the deep-sea mass spectrometer in conjunction with the photomosaics and physical sampling on this expedition to construct a geochemical map of key dissolved gases that can be overlaid on our biological and geological maps of the sites.