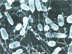
Acidic liquid carbon dioxide bubbles rise from the sea floor at NW Eifuku volcano. To avoid destroying camera lenses and plastic fittings on the remotely operated vehicle (ROV) Jason, the vehicle kept a safe distance from the rising CO2 bubbles. The inset (upper right) shows a few of the liquid CO2 bubbles, photographed from a safe distance. Click image for larger view and image credit.
Here we use the hydrothermal fluid and particle sampler (nicknamed "The Beast") to sample hydrothermal water and microbes at NW Eifuku on the ROV Jason Dive 196. Click image for larger view, more information and image credit.
NW Eifuku: Submarine Volcanoes and Global CO2 Increase
May 8, 2006
David Butterfield
Chemical Oceanographer
Joint Institute for the Study of the Atmosphere and Ocean Program, University of Washington
NOAA Vents Program, Pacific Marine Environmental Laboratory – Seattle,
Washington
![]() The CO2 Champagne vents at NW Eifuku. (Quicktime, 1.3 Mb.)
The CO2 Champagne vents at NW Eifuku. (Quicktime, 1.3 Mb.)
![]() Mussels and shrimp colonies at NW Eifuku. (Quicktime, 1.6 Mb.)
Mussels and shrimp colonies at NW Eifuku. (Quicktime, 1.6 Mb.)
![]() Scientists study the potential impact of increased CO2 levels in the marine environment. (Quicktime, 2.3 Mb.)
Scientists study the potential impact of increased CO2 levels in the marine environment. (Quicktime, 2.3 Mb.)
NW Eifuku, a relatively small and deep volcano by the standards of the Mariana arc, packs a chemical impact way beyond its size. It takes us into a pressure/temperature space where carbon dioxide (CO2) can exist as a liquid. CO2 is that newsworthy, global-warming molecule that we breathe and pump into the atmosphere at a phenomenal rate by burning fossil fuels. CO2 is a gas at room temperature and normal atmospheric pressure; and most people are familiar with dry ice, the solid form of carbon dioxide that quickly evaporates at room temperature. But, in order to observe the liquid form of carbon dioxide, you have to go to an environment of high pressure and cool temperature; in other words, the deep ocean environment.
Six gallons of CO2 gas at sea level will compress to about 3 tablespoons of CO2 liquid in the deep ocean. Liquid CO2 remains intact in droplets and does not mix with water, but at temperatures below about 10°C (50°F), and elevated pressure, water and CO2 form an ice-like hydrate. The physical/chemical properties of CO2 have given some people the idea that the deep ocean can be used as a place to get rid of the excess atmospheric CO2 from fossil fuel burning.
NW Eifuku is one of two places on earth where liquid CO2 has been observed — the other is the Okinawa Trough. About 60 m below the summit of NW Eifuku (summit depth is 1,535 m, or more than 5,000 ft), is an area of continuous venting of grape-sized droplets of liquid CO2 with a CO2 -hydrate "skin." From the carbon (and helium) isotope signature of the droplets, we can tell that there is a large component (about 90%) of ancient marine carbonate in the gas. This carbonate had to be subducted, then incorporated into magma (fluid that forms igneous rock, when cooled) that feeds NW Eifuku volcano, then "degassed" and cooled to form the CO2 -rich vents. The droplets come out of small cracks and crevices in the sea floor and rise up spinning and deformed through the water. Based on visual tracking of the droplets and properties measured in the water above the volcano, the droplets appear to dissolve over approximately 200 m of vertical rise. CO2 is an acidic gas and lowers the pH of seawater when it dissolves, favoring calcium carbonate dissolution.

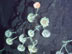
This is one of three sites where we sampled mussels and the surrounding water to understand the volcanic habitat. The shells of dead mussels dissolve very quickly, but the community maintains a high density. Squat lobster and shrimp are also abundant at NW Eifuku. Click image for larger view and image credit.
An issue of global importance is how increasing CO2 in the atmosphere and resulting lowering of surface ocean pH (increasing the acidity) will affect reef-forming corals and the marine plankton that produce oxygen and form calcium carbonate shells. NW Eifuku is an important "natural laboratory," where we can study the effects of increased dissolved CO2 on deep-sea life, and the effect of lowered pH from volcanic CO2 emission on the abundant mussel communities that live on rocky ridges near the acid-producing vents. It appears that the dissolution of shells is extremely fast, but the mussels are extremely abundant in spite of the challenging chemical conditions. It must be that this species has adapted to volcanic environments, but we are not sure how. Exploration of the oceans can produce spectacular observations and have an impact on global scientific and societal issues.
Sign up for the Ocean Explorer E-mail Update List.