Hydrates are ice with fuel inside - they can be lit by a match, or the application of any heat. Credit: Naval Research Laboratory. Click image for larger view.
Fire in Ice
Gas hydrates are a class of materials that Sir Humphrey Davy first described in the early 1800s. They since have been defined as "an ice-like crystalline mineral in which hydrocarbon gases and non-hydrocarbon gases are held within rigid cages of water molecules" (Sassen et al. 2001). Described in this way, gas hydrates may not sound very interesting or important, but they are. If you hold a hydrate nodule in your hand and light it with a match, it will burn like a lantern wick. There is fire in this ice.
Pain in the Pipeline - Frozen Resource and Frozen Hazard
People near the poles generally are familiar with gas hydrates because, like ice, glaciers and polar bears, they can exist on land at temperatures below freezing. They also can exist underwater above the freezing point at high pressures. When light natural gases (e.g., methane, ethane, propane, butane, pentane and their relatives) exist under either of these conditions, hydrates may form.
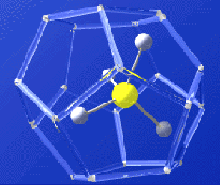
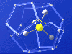
Gas hydrate molecule -- methane molecule locked inside watery cage. Credit: USGS. Click image for larger view.
In the early 1900s, the people of Russia considered hydrates to be a nuisance because they tended to clog up Siberian oil and gas pipelines. American oil and gas companies also have recognized hydrates as a hazard to offshore drilling activities. Not until the last decade, as oil companies pushed into deeper frontiers where vast reservoirs of hydrates exist, did the magnitude and global significance of this "fire in ice" come to light. Hundreds of millions of dollars in offshore lease tracts now under consideration overlap with known hydrate beds. A dangerous situation can result if a small amount of friction from a drill bit is applied erroneously. In fact, it could start a rapid and potentially catastrophic meltdown in a hydrate bed, which could harm drilling operators and the animals who rely on hydrates for habitat and food. Learn more about the potential hazards of gas hydrates in offshore drilling operations.
(top)
Fuel of the Future
In the past decade, ocean seismic surveys, deep-sea drilling and submersible studies provided the first hint at the amount of gas hydrates that exist on Earth. The concentration of gas molecules locked into hydrate crystals is up to 160 times greater than the same volume of pure gas. The result may be the largest single reservoir of carbon on the planet—at least twice as much as all other fossil fuels (e.g. oil, natural gas, coal) combined. The U.S. Exclusive Economic Zone (which extends to 200 m offshore) may contain as much as 200,000 trillion cubic feet of methane in hydrates (Kleinberg and Brewer, 2001). This is enough clean natural gas to power the United States for centuries. Read more about gas hydrates as a potential energy source.
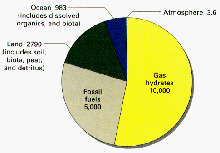
Distribution of organic carbon in Earth reservoirs (excluding dispersed carbon in rocks and sediments, which equals nearly 1,000 times this amount). More than half of the the carbon is locked up in gas hydrates. Credit: USGS. Click image for larger view.
Locating hydrates is one challenge; retrieving them is another. Mining them is not as easy as digging for coal or drilling for liquid. Several countries now are developing techniques to mine the gas locked inside of hydrates, as well as fuels that might be trapped beneath the ice layer. For the last several years, laboratory scientists have been working on ways to commercially produce methane hydrates. The separation of methane from the water molecules is relatively simple, but it is necessary to increase the temperature, decrease the pressure, or alter the reservoir’s chemistry by introducing anti-freeze. Nevertheless, many questions remain regarding this process of hydrate "dissociation" and the development of safe, economic means of gleaning methane from hydrates.
A Force of Nature
Based on some scientific studies, hydrates may join the legion of natural forces, such as orbital wobbles, mass volcanic eruptions and ocean circulation, that drive global climate changes. Methane, the main type of hydrocarbon in hydrates, is a powerful greenhouse gas. It traps heat as carbon dioxide—the most well-known and abundant airborne greenhouse gas. Past massive meltdowns of subsea hydrates may, for example, have released enough methane to drive up global temperatures by 5 to 10 degrees Celsius during the Late Paleocene era—some 55 million years ago. Such an increase in temperature today would be enough to melt the ice caps, raise sea levels by several feet, and transform America’s Wheat Belt into a desert. Learn more about global warming.
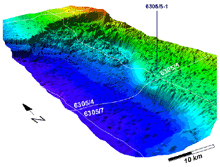
3D image of massive Storegga slide possibly caused by a gas hydrate melt-down -- resulted in a tsunami that drowned Scotland 7,000 years ago. Credit: www.ndwp.org. Click image for larger view.
Below 500 m in temperate and subtropical oceans, such as those found off the continental United States, hydrate beds may lie just beneath or above the sea floor over much of the continental slope. Geologists speculate that massive submarine "slumps", which can be likened to sea-floor avalanches, may occur when hydrates break away from the steep slope. Such massive slumps may drive the tidal waves that could drown miles of coastal shorelines.
(top)
Extreme Environment
As the new millennium dawned, a spacecraft hovered over Europa, a moon of Jupiter, and sent back images of an ice-covered world with possible vast oceans beneath. Just as life began in the Earth’s seas, it is possible that life exists in Europa’s ocean. Major funding initiatives have been launched in recent years to understand such extreme places, not only in the search for alien life, but also to mine potential new products from our own global seas. One species of heat-loving bacteria, for example, was found in a lake-floor hot-water vent and led to the discovery of an enzyme worth billions in genetic research.
Since 1987, NOAA-funded scientists have used research submersibles to explore the Gulf of Mexico and describe its geology, biology and chemistry at sea-floor oil and gas seeps. Like the dense communities that inhabit hot vents along the mid-ocean ridge, cold seep communities rely on the oil and gas seeping and venting from the sea floor for their primary source of energy and carbon. Tubeworm bushes, mussel and clam beds, and mats of giant bacteria are common surface indicators of substantial leakage.
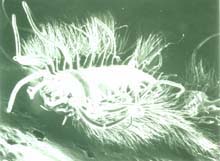
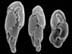
Micrograph of iceworm, Hesiocaeca methanicola, discovered living in a hydrate bed at 800 m depth during 1997 submersible dives in the northern Gulf of Mexico. Credit: C. Fischer. Click image for larger view.
In 1997, two scientists in a submersible pulled up to a block of gas hydrate exposed on the sea floor of the Gulf of Mexico. The surface of the block was moving, alive with a newly-discovered species of marine worm, now known as Hesiocaeca methanicola, burrowing into the ice. While such discoveries may result in new products or analogies to extraterrestrial life, they are even more important because they increase our knowledge of Earth’s complete "cast of characters." This is essential information for humankind because biodiversity — the variety of species, genes and ecosystems on the planet — is a critical indicator of global change and possible human impacts on the global environment. Read more about the discovery of polyacheate worms living on undersea gas hydrates.
References:
Sassen, R., ST Sweet, AV Milkov, DA Defreitas, and MC Kennicutt. 2001. Thermogenic vent gas and gas hydrate in the Gulf of Mexico slope: Is gas hydrate decomposition significant? Geol. 29(2): 107-110.
Kleinberg, R.L. and P. G. Brewer. 2001. Probing gas hydrates deposits. Amer. Scientist 89: 244-251.
Sign up for the Ocean Explorer E-mail Update List.