
By Tamara Frank - Halmos College of Natural Sciences and Oceanography, Nova Southeastern University
July 28, 2015

Glyphocrangon on the seafloor. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 3.4 MB).
I just read my colleague’s (Charles Messing) opening sentence in his log post, and I have one small addition: Working as a scientist on a deep-sea expedition offers numerous opportunities for both wonder and satisfaction and incredible frustration.
I have a very tech-heavy set up for doing shipboard electrophysiology, which means that numerous things can go wrong. The first problem was the tremendous electrical noise my electrodes were picking up. I’m working on the visual systems of some of these deep-sea crustaceans. As I explained in the background essay on vision, I do this by recording electricity generated by eyes when they absorb photons of light. The more photons they absorb, the bigger the signal. Big, however, is a relative term here, as the signal that I’m recording is in the microvolt range (.000001 volts), so I use an amplifier to amplify the signal 5,000 times.
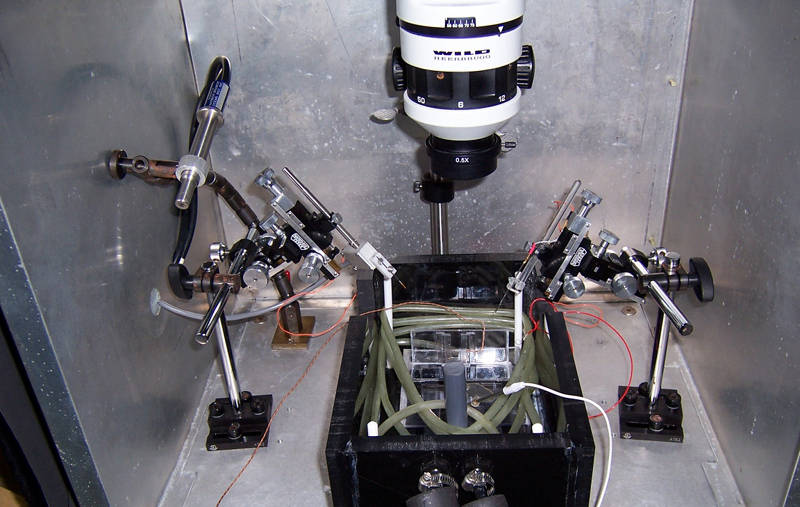
The silver Faraday cage is used to block out electrical noise. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.5 MB).
The problem with amplification is that it amplifies all electrical “noise,” including that generated by light and any equipment that’s turned on. To cut back on this electrical noise, I use a Faraday cage, the big metal cage shown in the picture, to block outside electrical noise, and ground the seawater bath in the black box as well, as that big pool of seawater can also behave like a big antenna. So, hypothetically, I’ve reduced the noise to about 20 uV, and should happily be able to record signals as low as 50 uV from eyes.
Unfortunately, I was getting noise in the millivolt (.001 volts) range, which would have swamped any biological signal that I was trying to record. I did all the usual tricks – switched out the amplifier cable, replaced the ground electrodes, replaced the recording electrode, replaced the wires going to the recording electrodes, turned off all the equipment I was using other than the amplifier to no avail.
I was just about to take a hammer to the amplifier, when my colleague Edie Widder wandered in and came up with a better suggestion. She immediately noticed that the foam padding that I had under the cage to reduce vibration (which the electrodes can also pick up) was wet with seawater. My black Plexiglas box was leaking, and the water was dripping through some holes in the back of the metal plate that the box was sitting on (hypothetically grounded as well). So while the surface of the plate wasn’t wet (which even I might have noticed eventually when it started dripping on my shoes), the padding under it was soaked. This served to turn the Faraday into one huge antenna due to something called a ground loop, the bane of every electrophysiologist’s existence.
Thanks to Edie, I finally got rid of the noise after my grad student and I laboriously took the set-up inside the Faraday apart and plugged the leaks in the box. So, problem #1 was solved, allowing me to see, sigh, all of the other problems I had yet to solve.
We were now collecting animals for our experiments (this being the royal “we,” as the remotely operated vehicle (ROV) pilots, Tony and Jamie, were doing all of the work), and animals that were collected with the suction sampler came up in excellent condition, but died overnight in my maintenance chambers (or, in this case, death chambers). I was the only one affected by this (of course) because everyone else worked on the animals as soon as they came up. Through process of elimination, I determined that it was the water that was being piped into the wet lab that was killing them, so we solved that problem by throwing a bucket over the side (again, the royal “we,” as my grad students did all of the work – it’s nice to be the queen) and collected water for long-term maintenance that way.
The first three animals that I worked on were all Glyphocrangon, which have large and lovely eyes that should be extremely sensitive to light. Turns out they are, and in spite of being most sensitive to blue light, apparently their eyes were being irreversibly damaged by the dim orange lights that we were collecting them under (problem #3). Jamie and Tony were thrilled when I asked them to switch to dim red light, because chasing mobile shrimp and crabs under barely visible dim orange light with a remotely operated arm from an ROV that was being dragged backwards was no longer enough of a challenge, and they were ready for something new.

Bathypaleomanella in bamboo coral. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.7 MB).

Bathypaleomanella in Iridogorgia coral. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 3.7 MB).
We were now at our deepest site, where Glyphocrangon weren’t present (or word go out that I was coming and they went into hiding), but there were some other shrimp species that I really wanted to work on, including Bathypalaemonella, which were always associated with a soft coral, most commonly the bioluminescent bamboo coral (above, left) or Iridogorgia (above, right) species. I thoughtfully selected a red shrimp hidden in a coral that would be really difficult to see in red light, because they weren’t active swimmers. However, it crawled deeper into the coral to escape the suction tube, and if they could be easily found under dim red light, where was the challenge for Tony and Jamie?
My colleagues also helped to make sure that Tony and Jamie never got bored, telling them that “sea cucumbers are easy to catch because they just sit there” (picture below on the left). They don’t – they can swim out of reach of the mechanical arm quite nicely (right image is sea cucumber rearing up for big escape) or “go ahead and open the Biobox – none of those animals can swim." That was actually true, but unfortunately, they floated quite nicely out of the BioBox as soon as it opened.

Sea cucumber resting on the seafloor. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 666 KB).

Sea cucumber making a quick escape. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 577 KB).
In spite of the challenges, Tony and Jamie managed to get some lovely crabs and Bathypalaemonella for me so that problem #4 could rear its ugly head. These collections were from 1,950 meters depth, and these animals couldn’t survive the pressure changes on the trip to the surface. The crabs were all dead, but I did get one Bathypalaemonella that gave me just enough data to suggest that they may have two visual pigments, like the two species of crabs that we studied in 2009, that also live amongst bioluminescent coral. Unfortunately , I didn’t get any more of this species, but hopefully the genomic analysis of this species in Heather Bracken-Grissom’s lab will show that they possess the machinery for two visual pigments. Her work doesn’t require dark collections, which means that she got all of the animals collected under light, and I got all the animals collected under dim red light.
With all the problems solved, we finally moved to a shallower site (1,000 meters depth), and I got a batch of crabs and shrimp to work on with only three days remaining in the cruise, meaning that I had to work 20 hours/day to finish up the last of my experiments. These specimens provided some very interesting data, including several species that may possess two visual pigments. So in the end, it was worth all of the problems (although it would have been worth it without the problems as well). I fortunately finished early enough that my grad student and I had the six hours we needed to pack up all the gear before we got back to the dock. I was sharing the lab with Sonke Johnsen and he (for some reason) wanted to pack his gear first, so we patiently waited 10 minutes as he unscrewed the two screws holding his camera stand to the benchtop and packed his camera gear in his suitcase.
Overall, the cruise was a tremendous success. We got a series of amazing videos and images, which we will continue to post on this website as we process them, thanks to the great cameras and pilots associated with the Global Explorer ROV. Over the coruse of the cruise, we: