
By Tamara Frank - Halmos College of Natural Sciences and Oceanography, Nova Southeastern University
Most deep-sea animals do not have color vision. They have a single, blue-sensitive, visual pigment because 1) as you go deeper through water in the ocean, all the colors disappear except for blue and 2) most bioluminescence is blue.
Why waste space in your eyes for multiple visual pigments when there’s only blue light to see? There are exceptions, of course, and that’s when things get really interesting. So far, we’ve discovered two species of deep-sea crabs that appear to have two visual pigments (see the Mission Plan to read about what purpose we think these serve). We say “appear” because studies of their visual physiology indicate that two visual pigments are there, one that’s primarily sensitive to blue light, just like we would expect, and a second one that’s sensitive to violet light (described below).
This is such an unusual discovery that other methods have to be used to verify our hypothesis that these species have two visual pigments. One way is to use molecular techniques (described below) to see if opsin genes are present for more than one visual pigment, i.e., an opsin that expresses the blue pigment and an opsin that expresses the violet pigment. The presence of both blue and violet opsins alone doesn’t prove that both visual pigments are expressed, but physiological studies demonstrating that crustaceans have two peaks of sensitivity together with molecular studies demonstrating the presence of two different opsins is pretty good proof that these animals have two visual pigments.
So, how do we do these experiments?
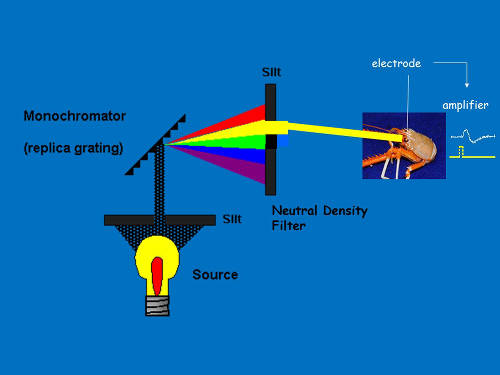
Figure 1. Diagram of monochromatic light. Image courtesy of the Bioluminescence and Vision on the Deep Seafloor 2015 expedition Download image (jpg, 202 KB).
These experiments can only be conducted on crustaceans (for this study, that includes crabs, shrimp, and isopods) that have been collected and brought to the surface in the dark (see Technology section).
Since we don’t speak “crustacean,” we have to get the crustacean’s eye to tell us what colors it can see. We do this by placing an electrode on the eye (we could do the same thing with you, using a contact lens electrode, if you’d let us), and recording the response from the eye when light is flashed on it. This response is generated by a large number of receptor cells in the eye that contain visual pigment. The visual pigment absorbs light and converts it to electrical energy, which is recorded by the electrode. The more light it absorbs, the bigger the signal.
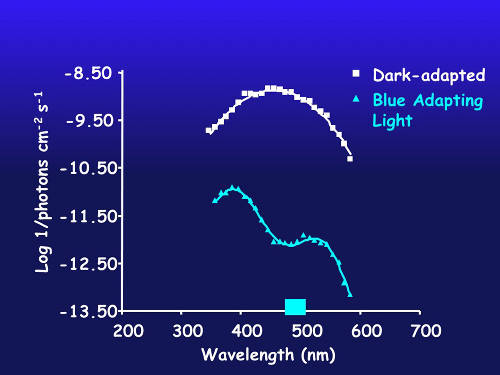
Figure 2. Spectral sensitivity of dark-adapted eye (white) and after chromatic adaptation with blue light (blue line) in a species with putative blue and violet visual pigments. Image courtesy of the Bioluminescence and Vision on the Deep Seafloor 2015 expedition Download image (jpg, 231 KB).
Since most deep-sea species have blue-sensitive visual pigments, flashing a blue light on the eye will produce a bigger signal than a yellow light of the same intensity, because the blue-sensitive visual pigment absorbs more blue light than yellow light.
Using a device called a monochromator (Figure 1) to split white light into individual colors, together with a neutral density filter, we can shine different colors on the eye, matched for intensity and based on the size of the response, determine what colors the animals see best.
Now, the problem is that, in crustacean eyes, if there are two visual pigments present, approximately 85 percent of the cells contain the blue-sensitive visual pigment and only about 15 percent contain the other one. The contribution of the blue-sensitive cells completely swamps any signal from the minority cells in the dark-adapted eye, so in order to tell if a second visual pigment is present, we have to do a chromatic adaptation experiment.
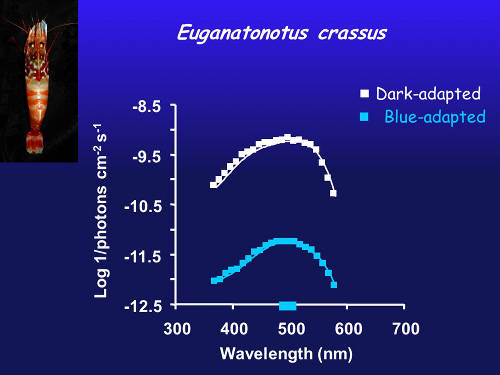
Figure 3. Spectral sensitivity of dark-adapted eye (white) and after chromatic adaptation with blue light (blue line) in a species with a single blue visual pigment. Image courtesy of the Bioluminescence and Vision on the Deep Seafloor 2015 expedition Download image (jpg, 246 KB).
A chromatic adaptation experiment involves shining a dim blue light on the eye for about half an hour, converting most (not all) of the blue visual pigment into a form that no longer absorbs light. With the blue cells no longer contributing very much to the response, the electrical signal from cells containing another visual pigment now become visible.
In Figure 2, the white curve is from the dark-adapted eye and demonstrates peak sensitivity to blue light. After adapting the eye to blue light, the contribution of violet-sensitive cells now dominate the response.
In an eye with a single visual pigment (Figure 3), shining a blue light on the eye decreases the sensitivity, but there’s still just a single peak of sensitivity, just like in the dark-adapted eye.
For this project, we will be using molecular methods to explore the visual systems (i.e., eyes) of deep-sea crustaceans. Specifically, we are interested in identifying the presence or absence of visual pigment proteins that allow the organisms to see light, and more specifically, bioluminescence.
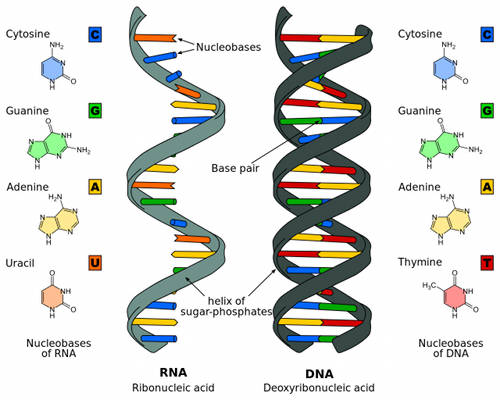
Figure 4. The differences between RNA and DNA. Image courtesy of the Bioluminescence and Vision on the Deep Seafloor 2015 expedition Download image (jpg, 465 KB).
As mentioned above, these visual pigment proteins are called "opsins" and previous studies have found evidence that some deep-sea crustaceans have opsins that can detect blue and violet light. The results from our research will allow us to identify the genes responsible for how organisms "see" in the deep sea. Vision and bioluminescence in the deep sea play important roles in the organism’s survival, including predator defense, feeding, and communication, so it is important to identify the molecular underpinnings of how these organisms “see.”
We use a recently developed molecular method called RNA-Seq (RNA sequencing) to study the eyes of deep-sea crustaceans. The term RNA-Seq refers to the sequencing of RNA from a specific tissue (for example, the eye) or organism (for example, a shrimp).
RNA (ribonucleic acid) is a nucleic acid (like DNA) and plays an important role in the coding, expression, and regulation of genes (Figure 4). Genes are the blueprints needed to make proteins. RNA is one of the macromolecules essential for all known life forms, and helps transmit genetic information from DNA to proteins within cells (Figure 5).
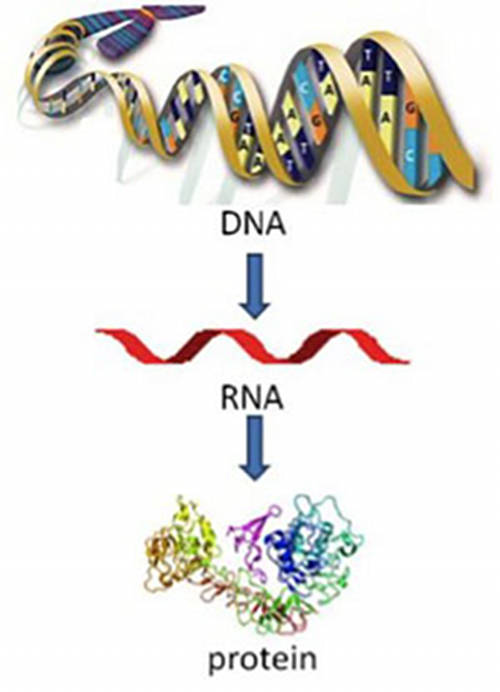
Figure 5. Flow of genetic information. Posted in Cancer 101 , Cancer Basics . Download image (jpg, 42 KB).
Sequencing the RNA of the eyes will allow us to identify the genes or proteins that play a role in vision and the detection of bioluminescent light. These results are also important to verify that animals with two peaks of sensitivity (as discussed above) have the opsins needed to make two different visual pigments.
What is great about this technique is that we can use animals that have been exposed to light and therefore can’t be used in the visual physiology experiments. Sometimes, during our collections, crustaceans will come up hanging on to corals or other structures, and therefore be blinded on the trip to the surface. Although their visual pigments are completely bleached out, their opsins are still intact, so we can still determine how many visual pigments they can make.
To sequence the RNA from an organism, we first need to isolate the RNA from the eye using RNA extraction kits in a molecular laboratory. Once the RNA is isolated, we will translate it into something called complementary DNA (cDNA). RNA needs to be "translated" into cDNA because cDNA is a more stable molecular form and this form can be "read" by the large sequencing machines.
Next, we make thousands of copies of the cDNAs so that they are in large enough quantities to be read on the sequencing machine. Once "read" by the sequencing machine, we have hundreds of sequence fragments that represent the genes that are present within the eye. The complete set of genes that are expressed within the eye can be called a transcriptome.
Finally, using bioinformatic tools, we can characterize the transcriptome to tell us which visual pigments (opsins) are present in the eye.