
By Dave Emerson - Bigelow Laboratory for Ocean Sciences
December 10, 2014
Zetaproteobacteria: Iron Mats at NW Eifuku. Video courtesy of Submarine Ring of Fire 2014 - Ironman, NSF/NOAA, Jason, Copyright WHOI. Video produced by Saskia Madlener. Music by Charlie Brooks.
Microscopic and SEM imagery courtesy of the Chan Lab and previously published in: Chan, CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ, 2010, Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation, ISME Journal, 5:717-727. Download (mp4, 62.8 MB)
Unfortunately the foremost reputation for bacteria is that of being germs, and while they can be dispassionate killers, the vast majority are harmless to humans. In fact, far more microbes provide beneficial services to our planet than are involved in causing disease.
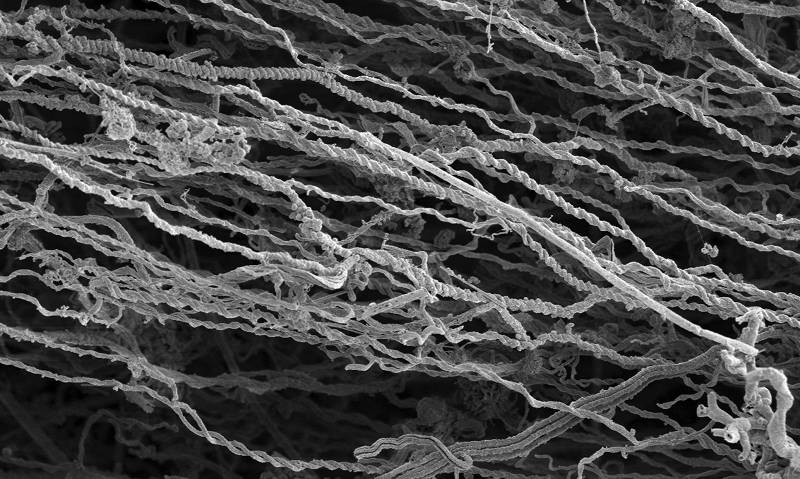
This is a scanning electron micrograph of a microbial iron mat showing all the threads of ferrihydrite mineral left behind by the iron-oxidizing bacteria. Note that there are no bacterial cells present in this image, just the mineral remains. Image produced by Sean McAllister and Clara Chan, University of Delaware. Download larger version (jpg, 484 KB).
One of these planetary services is the ability of microbes to either produce or alter minerals or rocks. By some estimates, over half the minerals on Earth are influenced by microbial activity. That is, if Earth were a ‘dead planet’ and life as we know it had not originated and evolved, the diversity of minerals and rocks that make up much of Earth’s surface would be far less.

Jason takes a sample of with the BioMat sampler at a location on NW Eifuku called Yellow Cone. Image courtesy of Submarine Ring of Fire 2014 - Ironman, NSF/NOAA, Jason, Copyright WHOI. Download larger version (jpg, 761 KB).
The iron-oxidizing bacteria we are seeking on this expedition offer a great example of how minerals and microbes interact. These bacteria convert soluble ferrous iron into ferric iron, a rusty precipitate. Chemically, this conversion is an oxidation reaction that releases a small spark of energy that the bacteria can capture for making a living. For a bacterium, making a living basically means producing more cells. The spark of energy released by this iron oxidation reaction is pretty small. If we compared it to the amount of energy a cell can acquire from sugar, it would be like comparing the energy from a single match to a nice fire in a fireplace. The iron mineral that results from this oxidation reaction is called ferrihydrite and it looks like rust, because, in a sense, it is rust. This is what microbial mats formed by iron-oxidizing bacteria are largely composed of: Lots of rust and a few cells.
Because the energy yield from iron is small, iron-oxidizing bacteria have to oxidize a lot of iron resulting in the production of a lot of ferrihydrite or rust. This creates a problem for them—how do they avoid becoming so coated in rust that they are cut off from their environment and no longer able to reproduce? The iron-oxidizers have evolved clever ways to avoid this problem by spinning long threads of iron oxides as they grow. The threads then become signatures for the bacteria themselves.
Different iron-oxidizing bacteria can produce different types of threads. Some make a helical stalk. The stalk is extruded by a single cell that grows at the end of its filament. When the cell divides the filament divides, so not only can a single cell be tracked by the filament it leaves behind, but its life history for at least a couple of generations can be followed, too.
Other iron-oxidizers grow as rod-shaped filaments of cells that surround themselves with a tubular sheath also composed of ferrihydrite. In this case, a dozen cells or more may contribute to the production of an individual sheath. There are other groups that produce other types of filamentous structures all made of ferrihydrite.
One of the challenges we face as scientists is telling what species of iron-oxidizer is producing what type of filament and how that filament might be advantageous for that species within the iron mat.
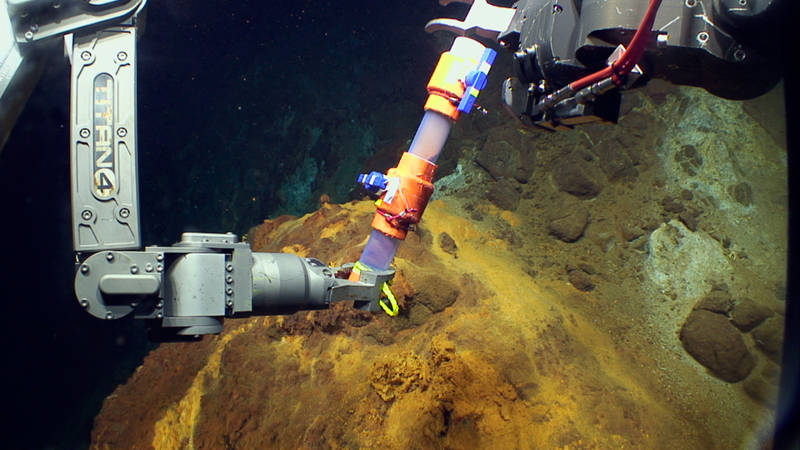
The Jason manipulator arms use a Scoop sampler to collect iron-oxidizing microbial mat at NW Eifuku. Image courtesy of Submarine Ring of Fire 2014 - Ironman, NOAA/PMEL. Download larger version (jpg, 859 KB).
Perhaps the most amazing part of having lots of these bacteria living together, each spinning their own threads of iron, is that the end result is a cohesive microbial mat structure woven together by all the different threads. Perhaps this is more than just a way for the cells to prevent themselves from becoming encrusted in iron. The structure of the mat helps to control the flow of hydrothermal vent water that contains the iron they need for growth and it provides a stable place for the bacteria to live. As the mat grows, it also provides a substrate for other bacteria to attach to and grow in the vent fluids. In some cases, these could be other iron-oxidizing bacteria or other microbes that can live on other constituents of the vent fluids. The mat then becomes a microbial community.
So what happens to these iron mat communities that are woven from ferrihydrite when the hydrothermal vents that sustain them turn off? We don’t fully know the answer to this question, but one scenario is that the ferrihydrite minerals become more compacted after the vent turns off. Eventually, through time and pressure, the mat is converted to other mineral forms of iron that are denser and more stable than ferrihydrite.
Ultimately the densest, most stable iron mineral is hematite. If you stubbed your toe on a piece of hematite you wouldn’t call it a microbial mat, you would call it a rock. Hematite is the most sought-after iron ore. Between two and three billion years ago, the large deposits of hematite that produce almost all the iron we use today to make steel were deposited in ancient seas. Could these have started out as microbial iron mats fed by what at that time was an iron-rich ocean? These are some of the questions we hope to answer by studying these modern iron mats along the Ring of Fire on this expedition.