
By Jeffrey Seewald, Senior Scientist - Woods Hole Oceanographic Institution
Locating a hot-spring on the seafloor can be like searching for a proverbial needle in a haystack. Considering that hot-spring activity is restricted to relatively small areas along the 80,000 kilometers (km) of the global ridge system, it is a challenge to locate these systems in water depths that can approach 5 km. Fortunately, when hydrothermal fluids are released at the seafloor, they mix with cold seawater and form expansive plumes that rise several hundreds of meters before spreading laterally. These plumes are substantially larger in size than the narrow chimney-like structures from which the hydrothermal fluids emanate.
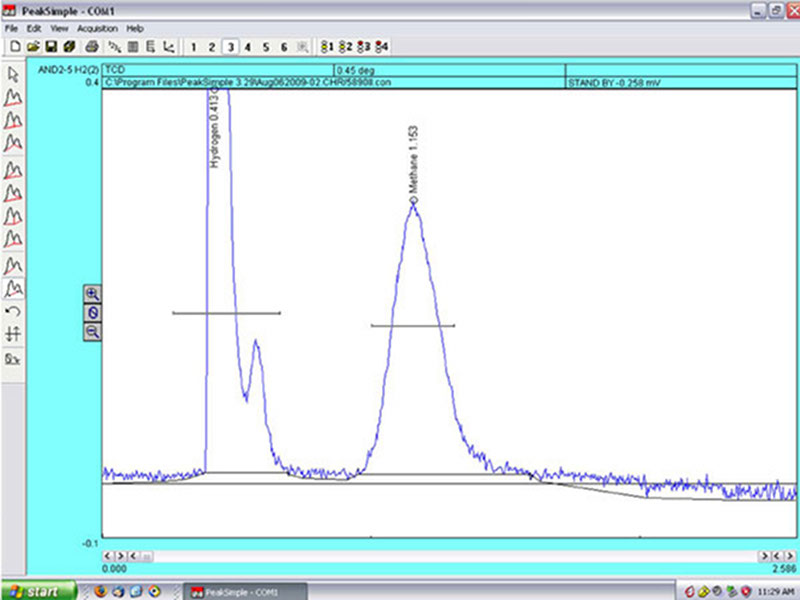
The gas chromatograph generates a graph with two peaks, which show the relative amounts of hydrogen and methane contained in the water sample. Image courtesy of NOAA Okeanos Explorer Program, MCR Expedition 2011. Download larger version (jpg, 1.2 MB).
Because hydrothermal fluids contain abundant dissolved metals and gases, the plumes they create are chemically distinct from surrounding seawater and represent chemical anomalies within the water column that we can detect using modern analytical techniques. One means of detection takes advantage of the fact that hydrothermal fluids are characterized by methane abundances that may be more than a million times more concentrated than in typical seawater, and that we are able to measure exceedingly low concentrations of methane. Thus, by systematically collecting seawater samples in areas of suspected hydrothermal activity and measuring the concentration of methane, we can identify hydrothermal plumes that tell us whether we are in close proximity to an area of hydrothermal activity.

Jill McDermott, PhD student at Woods Hole Oceanographic Institution, uses the gas chromatograph to determine the hydrogen and methane content of a water sample in the Seewald lab. Image courtesy of NOAA Okeanos Explorer Program, MCR Expedition 2011. Download larger version (jpg, 4.2 MB).
The instrument used to measure the abundance of methane in seawater is referred to as a gas chromatograph. This is a relatively simple device in which a mixture of gases can be separated before each gas is passed through a detector that measures the gas’ abundance. Separation of the gases is accomplished by injecting a sample into a continuously flowing stream of nitrogen that travels through a six foot long 1/8 inch diameter stainless steel column filled with a mineral known as a zeolite. The zeolite is characterized by microscopic pores through which the gases must travel to exit the column at the other end. Owing to variations in the molecular size of different gases, they pass through the column pores at different rates, and are separated from each other by the time they reach the detector at the end of the column. Because the rate at which gases pass through the column increases with increasing temperature, it is housed within an oven so that the time required for the gasses to pass through the column can be regulated.

The sample gases are carried in a nitrogen gas flow through a column filled with zeolite minerals, seen here as six foot stainless steel tube coiled in two loops inside the instrument. The gases have different molecular sizes, so the smallest gases pass through the microscopic pores in the zeolite more quickly than larger gases. This separates the gases and allows detection of each individual gas species at the end of the column. Image courtesy of NOAA Okeanos Explorer Program, MCR Expedition 2011. Download larger version (jpg, 5.3 MB).
There are numerous types of detectors that can be used to detect gases of varying composition. For the case of methane, we take advantage of the fact that it is flammable and use a flame ionization detector. This device consists of a small flame and an ammeter that measures the quantity of ions that are generated when a peak of methane is burned after exiting the column. Because the number of ions increases with the amount of methane, the response of the detector provides quantitative information regarding the abundance of this gas. The flame ionization detector is extremely sensitive, allowing very small quantities of methane to be measured.
Chemical analysis of methane dissolved in seawater has one complicating factor that must be dealt with before making the measurement. Because water cannot be injected into the gas chromatograph, the gas must first be removed from the seawater. In the case of methane, we add nitrogen gas to a syringe containing our seawater sample. This causes the methane to leave the seawater and enter the overlying nitrogen gas-filled space of the syringe. The process is analogous to a can of soda “going flat” after it is opened. We then inject the overlying gas into the gas chromatograph for analysis.

A sample has just been injected into the gas chromatograph using the syringe. The hydrogen has passed through the column and detector and is visible onscreen as a peak in the graph. Since it is a larger molecule than hydrogen, the methane peak will appear next. Image courtesy of NOAA Okeanos Explorer Program, MCR Expedition 2011. Download larger version (jpg, 5.3 MB).
The gas chromatograph is a sturdy instrument that can be rapidly set up in ship-board laboratories, allowing these measurements to be made at sea. This is extremely helpful during exploration of the deep ocean since results are immediately available and can be used to guide the search for areas of venting at mid-ocean ridges. Once a plume is located based on anomalous methane concentrations, it is repeatedly sampled to find the location of maximum methane abundance. Increasing concentrations are interpreted as a compelling indicator that the plume source is being approached, and a new area of hydrothermal activity must not be far away.