
By Mingshun Jiang, Associate Research Professor - Harbor Branch Oceanographic Institute at Florida Atlantic University
June 1, 2017
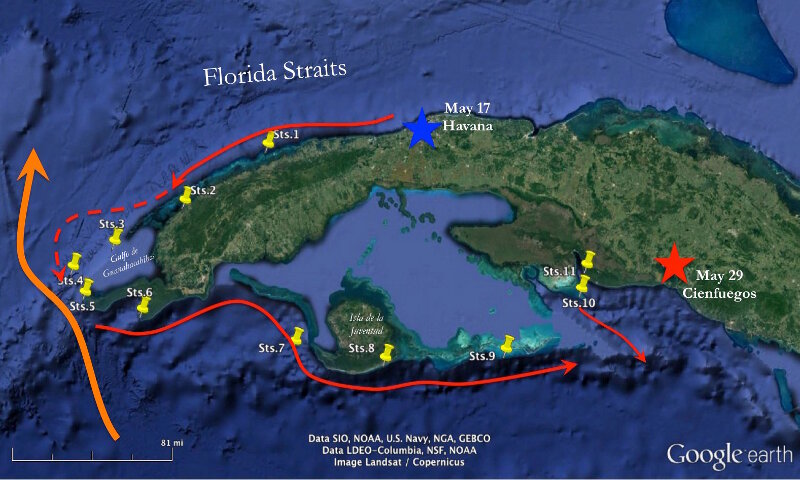
Figure 1. Map of expedition survey sites (yellow pins). Broad orange arrows indicate the offshore Yucatan Current, while the red arrows indicate the shelf break countercurrents. Image courtesy of Cuba’s Twilight Zone Reefs and Their Regional Connectivity. Download larger image (jpg, 329 KB).
On a sunny Tuesday afternoon, I took a flight from Miami to Havana to study deep corals in Cuban waters for the first time. Once arriving at Havana, I joined my colleagues on board the R/V Walton Smith to embark on a scientific cruise circling the western Cuban Island, with the first leg starting from Havana on May 17 and ending at Cienfuegos on May 29 (Fig. 1).
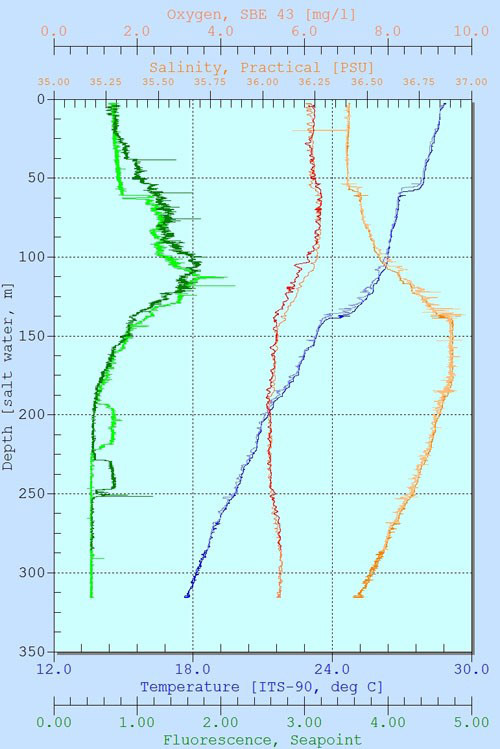
Figure 4. Depth profile of tempeature (oC), salinity (psu), fluorescence (equivalent to chlorophyll a), and dissolved oxygen (mg/L) concentrations at Station 9 (May 27). Two lines of similar colors for each parameter are due to downcast and upcast, respectively. Note there is a background value of fluorescence ~ 0.5 is likely reflecting other materials such as colored dissolved organic matter (CDOM), not chlorophyll. Image courtesy of Cuba’s Twilight Zone Reefs and Their Regional Connectivity. Download image (jpg, 88K B).
Unlike my colleagues, however, my goal is not to find out what kind of corals or fishes live in these areas. Rather, the questions I hope to answer are: what is the ocean environment these corals live in and what are the physical forces that control these water properties? In particular, I hope to measure the carbon dioxide (CO2) concentration and pH values along with currents and other environmental parameters near the reefs. This information is important because because the CO2 tends to be over-saturated in these deep waters and therefore the pH value is normally lower than surface waters. If the pH value is too low, then corals may have a hard time calcifying or reproducing. The reef structure may also be eroded due to high dissolution rates. The information about currents will tell us where these waters are from and where they may go.
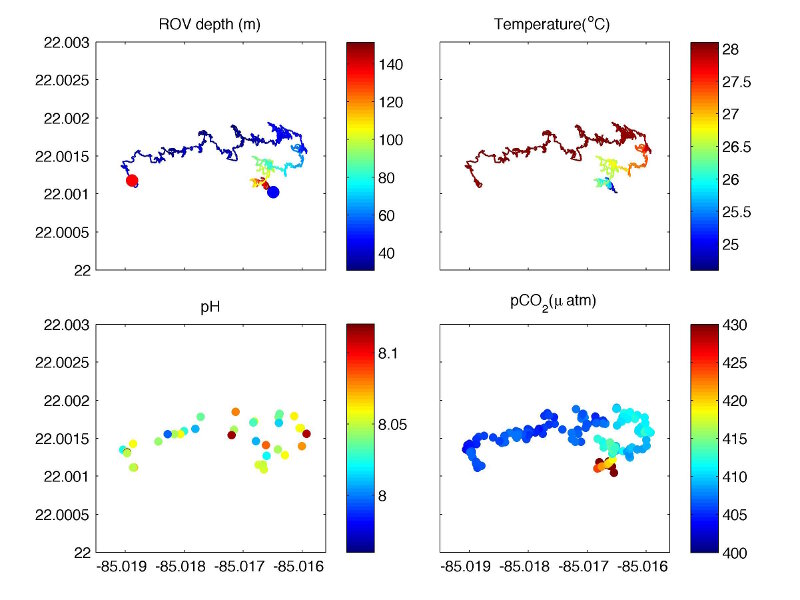
Figure 5. ROV track and the measured parameters from dive 415 on May 21: (top left) depth (m), (top right) temperature (oC), (bottom left) pH, and (bottom right) pCO2 (µatm). Blue and red dots indicate the start and end of the dive. The ROV first dove to around 150 meters at the base of the reef (blue dot in the top left panel), slowly climbed the reef wall and then moved around on top of the reef. Spatially the ROV covered about 300 meters in the west-east direction and less than 100 meters north-south over a period of ~ 3 hours. Image courtesy of Undersea Vehicles Program, University of North Carolina Wilmington, Cuba’s Twilight Zone Reefs and Their Regional Connectivity. Download larger version (jpg, 343 KB).
To answer these questions, my colleagues Jason White and Lance Horn helped me to put three sensors on the Mohawk remotely operated vehicle (ROV): a Seabird FastCat CTD, a SAMI pH, and a HydroC pCO2 (Fig. 2). When the ROV is hovering closely above the reefs, these sensors will collect key data about the living environment of the coral communities. The sampling rates vary from one per second (CTD) to one per one to two minutes (HydroC, SAMI). Between the ROV dives, we also cast a CTD rosette down to near the bottom to measure the profiles of water properties (Figs. 3, 4). At the same time, a number of Niskin bottles are hung on the rosette to collect water samples at different depths to measure the CO2 and alkalinity concentrations. Alkalinity is a parameter measuring the concentration of bases (mainly bicarbonate), which are used to combine with calcium ions to form the hard shell of corals and other shell-forming organisms.
The results were mixed. For the most parts, they were what we expected: A healthy environment with warm and salty water (temperature between 22-29 oC or 70-85 oF), sufficient dissolved oxygen, and relatively low, but still normal, pH.
The results, however, carried two surprises. First, at every deep station (>150 meters), there was a deep chlorophyll maximum that could reach as deep as 120 meters (Figure 4). This indicates there are significant amounts of algae at this depth, which are an important food source for corals and other organisms. Secondly, the pH value over the reefs varied significantly within a few tens of meters even though temperature, salinity, or CO2 concentration showed little spatial variations (Figure 5). This would mean that alkalinity, which also affects pH, also changed significantly over the reefs. One possible explanation is the spatial variations of benthic communities as we have seen. In areas dominated by corals, a large amount of bicarbonate is used for calcification, which also lowers the pH. This does not happen in areas dominated by sponges or algae. But is the measured pH correct? To answer this, we will have to wait until my colleague Leticia Barbero at NOAA's Atlantic Oceanographic and Meteorological Laboratory (AOML) directly measures the pH and alkalinity of these water samples in the lab.

Figure 2. Mohawk ROV with the additional sensors attached for this expedition. Image courtesy of Undersea Vehicles Program, University of North Carolina Wilmington, Cuba’s Twilight Zone Reefs and Their Regional Connectivity. Download larger version (jpg, 350 KB).

Figure 3. Denis Ilias, University of Miami, Marine Technician is launching the CTD rosette. Image courtesy of Cuba’s Twilight Zone Reefs and Their Regional Connectivity. Download image (jpg, 107 KB).