
By Brenna Hays - Graduate Student, Nova Southeastern University
July 21, 2015

Figure 1: Dr. Charles Messing identifying collected specimens. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 200 KB).
I knew going into grad school that I wanted to learn as much as I could about marine invertebrates. Dr. Charles Messing, a prominent invertebrate taxonomist, has graciously allowed me to learn from him and brought me on this research trip. I don’t have any experience or background in bioluminescence, but I’ve always found it extremely interesting (who doesn’t like to look at pretty twinkling lights?).
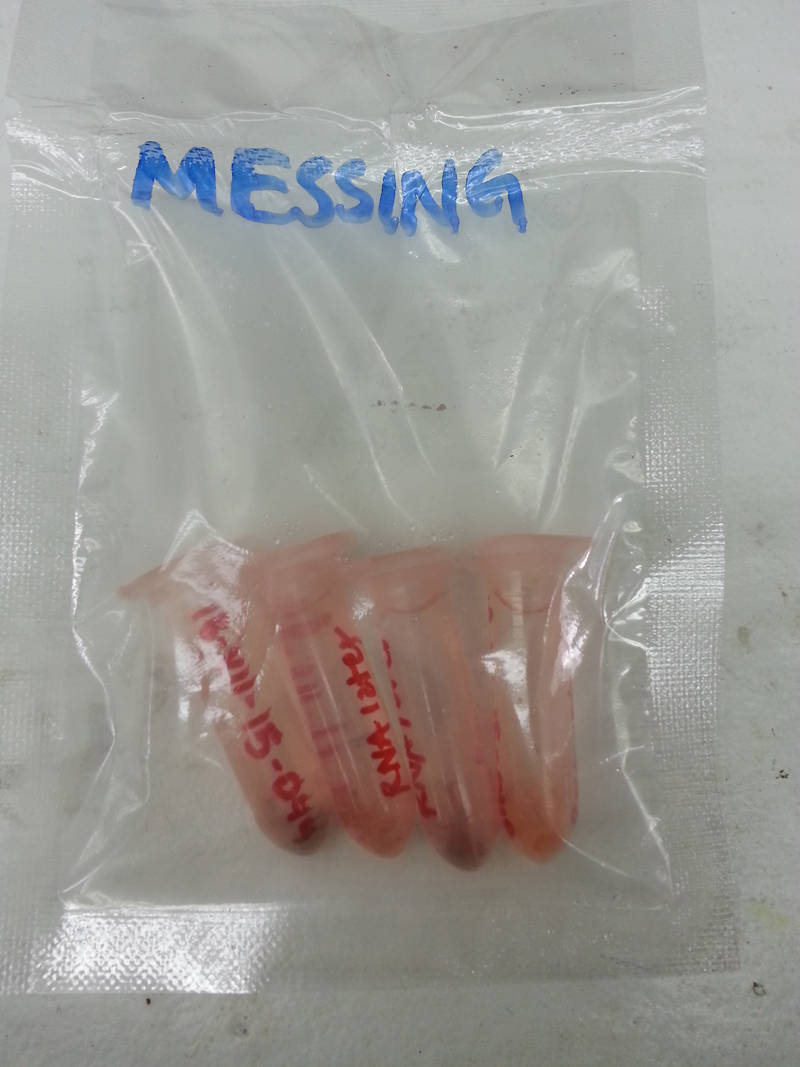
Figure 2: Example of some specimen bits preserved in RNA later for genetic profiling. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.2 MB).
I am here on Research Vessel Pelican as part of the “preservation posse” with Dr. Messing. Our job, once the poking and prodding are completed, is the taxonomic identification and preservation of all of the specimens collected on each dive (aside from the crustaceans that will be extensively studied by Dr. Frank and Dr. Bracken-Grissom).
For each specimen, we begin with visual identification (first by eye and then, if needed, with a dissecting microscope; see Figure 1). The specimen is identified to the lowest taxonomic level possible with some confidence, then catalogued and readied for preservation.
First, a small piece of the specimen is cut up into very small pieces and placed into a vial containing RNA Later (Figure 2). This preservation liquid is an RNA stabilizing reagent that will stabilize the RNA in the specimen’s tissue samples and allow genetic profiling at a later date. Once the pieces settle to the bottom of the vial (this usually take about a day), the vials are frozen and will be sent out for genetic profiling when we get back home.

Figure 3: Example of some specimens preserved in 70% ethanol. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.3 MB).
Another slightly larger piece of the specimen is placed into a vial containing 70 percent ethanol. Ethanol is used to preserve organisms like crustaceans, echinoderms, and corals (Figure 3). Ethanol keeps DNA intact for genetic processing and, since it is not nearly as toxic as other preservation liquids, is an excellent method when keeping specimens for collections or for use as teaching tools.
The third and final preservation liquid we use is 10 percent formalin. Although it can be extremely toxic and must be used out on the deck of the ship, it is quite efficient in keeping the coloration and tissues of the specimens intact, especially when dealing with soft-bodied organisms that do not hold up in ethanol (Figure 4). We use durable plastic bags to hold the formalin specimens and heat-seal them for leakage prevention (Figure 5).

Figure 4: Iridogorgia octocoral preserved in 10 percent formalin. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.5 MB).

Wooden portions of this octant or sextant are now largely consumed but the brass pieces are in situ and articulated. Prior to use of the Global Positioning System (GPS), ships used celestial navigation, measuring the angle between the Earth’s horizon and planets, stars and the sun, to find their position nd navigate the sea.Figure 5: Dr. Messing heat sealing a formalin preserved specimen for transport. Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download larger version (jpg, 1.3 MB).
My thesis project focuses on the skeletal morphology of a particular family of crinoids, so I was really hoping we would be able to collect some. Crinoids are the sea lilies and feather stars; they are echinoderms—cousins of sea stars and sea urchins. We finally succeeded yesterday in collecting three feather stars, two of which I may use in my research (yay!!).

Figure 6: Possible new sighting of Thaumatocrinus jungerseni (ID source: Clark & Clark, 1967). Image courtesy of NOAA Bioluminescence and Vision on the Deep Seafloor 2015. Download image (jpg, 70 KB).
The other specimen belongs to a genus (Thaumatocrinus) that has never been reported from the Gulf of Mexico before. In fact, the only records of this genus from the entire Atlantic Ocean are two samples of the species Thaumatocrinus jungerseni taken south of Iceland. Unlike all other feather stars, species of Thaumatocrinus have 10 undivided feathery rays; virtually all other feather stars have five rays that usually divide at least once to produce 10 or more arms. We will examine the specimen further when we return home to determine whether it is T. jungerseni or, possibly, a new species!
This afternoon concluded dive #10 on the Pelican, and so far we have catalogued around 120 specimens. These include 14 different species of decapods, two species of amphipods, several branching octocorals, sea whips, sea pens, anemones, brittle stars, sea cucumbers, and a sea star. From octopus sightings, to swimming cucumbers and salt domes of extraterrestrial proportions, this trip has definitely been a highlight of my graduate career.