
By Sönke Johnsen - Professor of Biology, Duke University
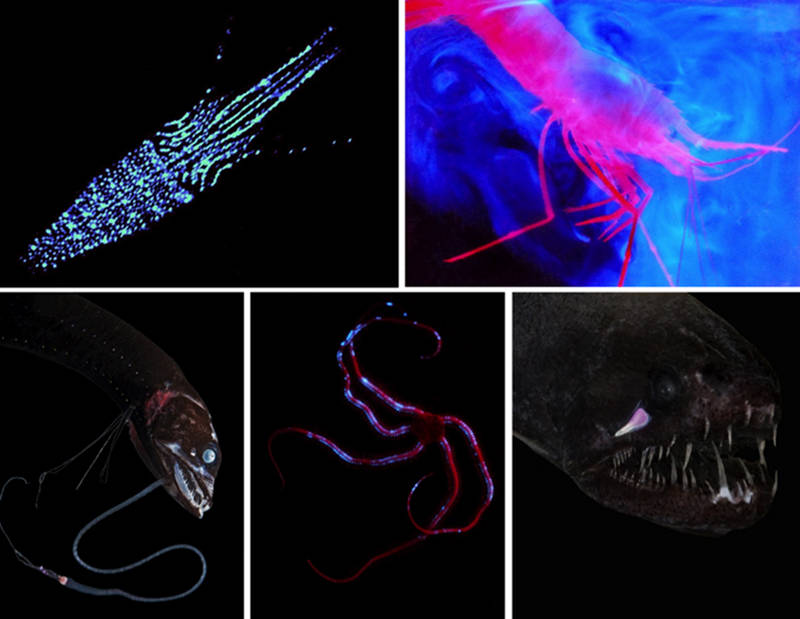
Figure 1: Examples of the various uses of bioluminescence. Upper left) Counterillumination of the bottom surface in the eye-flash squid, Abralia veranyi. Upper right) Defensive spew bioluminescence in the shrimp, Parapandalus. Lower left) The bioluminescent lure of the barbeled dragonfish, Eustomias pacificus. Lower middle) Possible warning bioluminescence in the brittle star, Ophiochiton ternispinus. Lower right) Bioluminescent searchlight under the eye of the threadfin dragonfish, Echiostoma barbatum. Image courtesy of the Bioluminescence and Vision on the Deep Seafloor 2015 expedition. Download larger version (jpg, 295 KB).
In addition to being beautiful, bioluminescence is common and almost certainly important in the open ocean. Depending on how you count it, around 80 percent of the animals in the water column (i.e., not on the ocean bottom) emit light. Bioluminescence is most common among fish, squid, and what we call the gelatinous zooplankton – jellyfish, siphonophores, comb jellies, and other animals that are mostly made of water.
While usually blue in color, because this is the light that travels best through the water, bioluminescence can range from nearly violet to green-yellow (and very occasionally red), and is emitted in three different ways:
Regardless of whether it is vomited, made in cells, or made by bacteria, the light is created by the same basic reaction. In this reaction, a small organic molecule – called the substrate – is oxidized. The reaction requires energy and is helped along by an enzyme.
There are about four different substrates in marine organisms, all of which are referred to as luciferins, the same name that we give to the substrate in fireflies. The enzyme is called a luciferase, again the same name used in fireflies. Mix these ingredients together, either in a cell or in the water itself, and you get light.
However, while we understand some of the chemistry, much about bioluminescence remains a mystery. First of all, we can mostly only guess at its functions. A few functions are fairly well understood. For example, we know that the large number of small light organs on the lower surface of many fish, shrimp, and squid are there to hide the silhouette of the animal when it’s viewed from below (Figure 1, upper left). This is called counterillumination.
We also are reasonably certain that the stalked light organs in certain deep-sea fish are lures for prey and in some cases for potential mates (Figure 1, lower left). And we know that the light organs under and over the eyes of certain fish are used as searchlights (Figure 1, lower right).
Most bioluminescence, however, seems to only occur when the animal is being touched, and we are still not entirely sure what the purpose of this is. We think it protects the animal in a number of ways, including blinding or startling an opponent, misleading them by detaching a glowing body part, or possibly attracting animals that eat the opponent. However, as is so often true in science, we have a long way to go before we can confirm these ideas. We also suspect that some deep-sea animals are using bioluminescence to communicate with each other, but are only certain of this in a very small number of species.
Another open question in bioluminescence research is: why is it distributed the way it is? It is very common in the water column of the ocean, less common on coral reefs and other places near the shore, rare on land, and nearly non-existent in freshwater. We really have no idea why this is the case. For example, why is bioluminescence on land mostly limited to a few insects and mushrooms? It is of course dark in the deep sea, but it’s equally dark at night on land.
The near lack of bioluminescence in freshwater is especially puzzling. Yes, the oceans are much older than most lakes and so bioluminescence has had more time to evolve, but our best guess is that bioluminescence has evolved at least 40 times, so one would think that it would have evolved at least once in one of the larger and deeper lakes, like Lake Baikal.
A final question is: how did bioluminescence evolve? Light in a dark place is obviously useful, but did bioluminescence start out as a useful optical trick or did the emitted light serve another function?
One hypothesis is that bioluminescent reactions originally helped get rid of oxygen. Oxygen, while necessary to us, is also harmful, because in a very real sense it can burn the insides of our cells by oxidizing molecules. This is why we are told to eat blueberries and other such foods, because they are full of antioxidants – molecules that grab oxygen and carry it away safely.
It turns out that luciferins are all excellent antioxidants, and so one idea is that the emitted light was a safe way to get rid of the energy of the oxidation itself. Instead of the energy causing damage, it just floats away in a puff of blue light. Like so many ideas about bioluminescence, this clever idea still needs to be proven.
Bioluminescence is a subject with many more questions than answers!