
By: Monica Heintz - University of California – Santa Barbara
Methane (CH4) (figure 1) is the simplest hydrocarbon, and is the primary component of the natural gas that we burn for energy. Methane is also an important greenhouse gas: it has about 23 times the global-warming potential of carbon dioxide when it is released into the atmosphere. There are many sources of methane. Some methane comes from human activities; landfills, rice cultivation, and ruminant farm animals (like cows) are all large methane sources. Other large methane sources, like the ocean and wetlands, are natural.
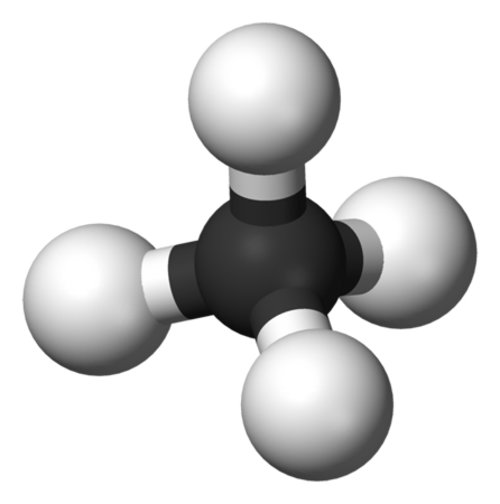
Figure 1. Methane is composed of one carbon atom surrounded by four hydrogen atoms. It is the simplest hydrocarbon. Image courtesy of INSPIRE: Chile Margin 2010. Download image (jpg, 814 KB).
Methane is produced in two primary ways in the ocean, thermogenically and biogenically. Thermogenic methane is produced deep within the seafloor when organic material is degraded by the earth’s heat. Biogenic methane is produced as a waste product when microorganisms called methanogenic archaea eat organic material; these microorganisms can only produce methane in reducing environments.
Thermogenic and biogenic methane are chemically identical, although their stable isotopic signatures vary. Stable isotopes are molecules which vary in the number of neutrons they have. The common stable (non-radioactive) carbon isotopes are carbon 12 (12C – 6 neutrons) and carbon 13 (13C – 7 neutrons). More of the carbon in biogenic methane is 12C, while thermogenic methane tends to contain comparatively more 13C.
This characteristic allows scientists to distinguish between these methane sources in order to learn something about the environment of methane formation. Both thermogenic and biogenic methane have relatively less 13C than other carbon sources. This characteristic allows scientists to track carbon that is derived from methane.
Much of the methane (greater than 80%) generated within today’s ocean is consumed by microorganisms before it reaches the atmosphere. In this way, microbes help the earth retain its heat balance. There are two groups of microorganisms that eat methane, they are both called methanotrophs, but there are some very important differences. Archaeal methanotrophs are only able to consume methane where there is no oxygen available (for instance, seafloor reducing ecosystems and water column oxygen minimum zones) and must have a bacterial partner who consumes sulfate (figure 2a). Bacterial methanotrophs (figure 2b) are only able to consume methane where there is oxygen available (most of the water in the ocean, and the very top layer of sediment), and act independently. Archaea and Bacteria are both single-cell organisms, but they represent two separate branches in the tree of life.
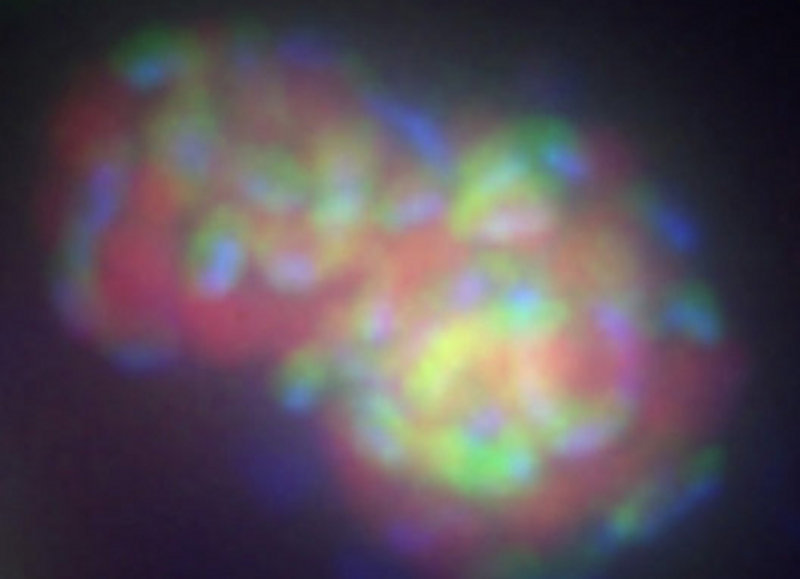
Figure 2a. A cluster of anaerobic methane-consuming archaea (red) and sulfate-reducing bacteria (green). This cluster of cells has been labeled with two ribosomal rRNA probes that target these two specific groups and have fluorescent molecules attached that show up as different colors when viewed with a special microscope. Image courtesy of Victoria Orphan, California Institute of Technology . Download larger version (jpg, 99 KB).

Figure 2b. A seafloor blanket of chemosynthetic communities on a methane hydrate-associated mound on the seafloor in the Santa Monica Basin. The white and orange microbial mats contain aerobic methanotrophs, along with other chemosynthetic bacteria. The space between the mats is crowded with clams. Image courtesy of Monica Heintz. Download larger version (jpg, 340 KB).
In addition to the important job of keeping methane from the ocean out of the atmosphere, methanotrophs make carbon available to higher trophic levels. Some very small sea creatures are able to feed directly on methanotrophs, this is called grazing. Other, highly specialized animals, such as mussels (figure 3) and some tube worms, keep a population of methanotrophs in their bodies. This is an example of a symbiotic relationship, where the mussel or worm gets some carbon from the methanotroph and the methanotroph gets a comfortable home from the animal. Both in the case of grazing and in the case of symbiosis, the incorporation of carbon from methane can be tracked based on its stable isotopic signature.

Figure 3. A close up of a group of bathymodiolin mussels from a methane seep. These mussels have the ability to harbor both sulfide-oxidizing as well as methanotrophic bacterial symbionts within their gills. Image courtesy of CRROCKS/NSF. Download larger version (jpg, 1.9 MB).
Although little methane from today’s ocean makes it to the atmosphere, there have been times in the geologic past when it appears that large releases of methane have overwhelmed the capacity of microbial methane consumers, causing a large emission of methane from the ocean to the atmosphere. Methane hydrates on and within the seafloor (figure 4) are a natural, climate sensitive, methane pool, and may have been the culprit in some past periods of increased methane release and resultant global warming.

Figure 4. This is a methane hydrate mound on the seafloor. The bubbles show that methane is continuously leaking from features like this. If bottom waters warmed, this entire feature may be destabilized and leak methane at a higher rate. Image courtesy of INSPIRE: Chile Margin 2010. Download image (jpg, 66 KB).
Methane hydrate is formed when water molecules form an ice cage around a methane molecule (figure 5). In areas with the right balance of pressure (high), temperature (low), and methane concentration (high), methane occurs in this form, and may be stored over very long periods of time. However, if temperature or pressure conditions change, the hydrate may destabilize and release methane to the surrounding environment. For this reason, it is important to understand the marine methane cycle, so we can make predictions about how it may change as our climate begins to warm.
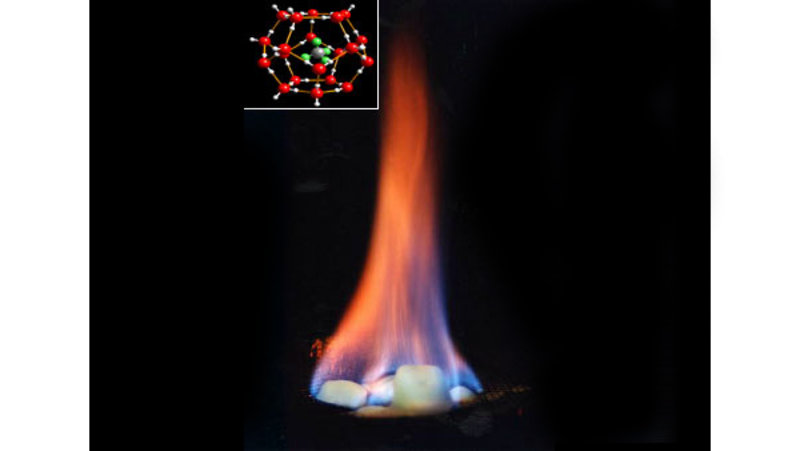
Figure 5. Methane hydrate will burn when lit. The inset image shows the structure of methane hydrate; the green and grey molecule in the center is methane and the red cage is the ice structure. Image courtesy of INSPIRE: Chile Margin 2010. Download image (jpg, 25 KB).